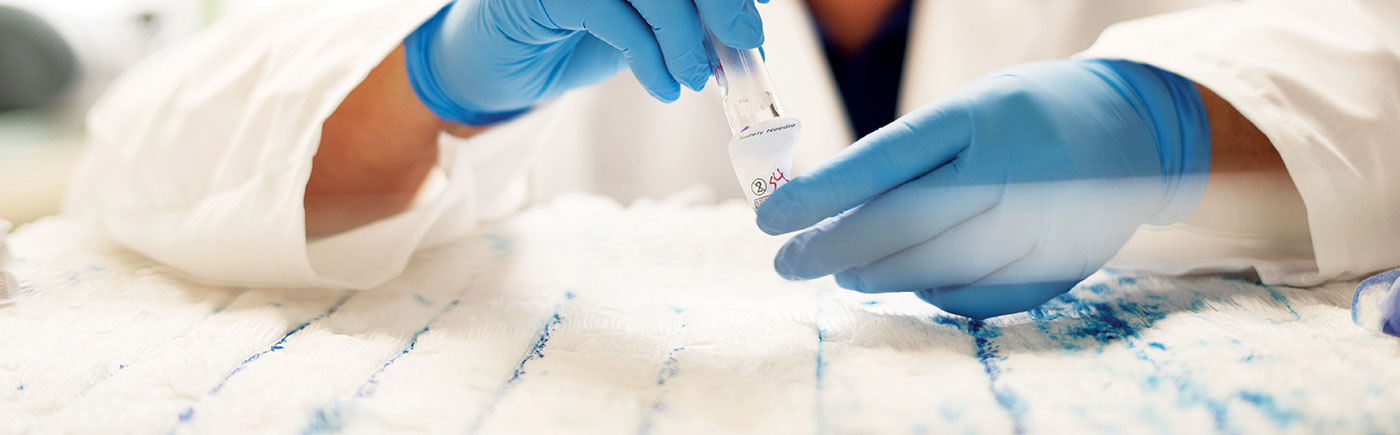
One-stop expertise in medical device testing
Partner with us and gain a reliable medical device testing lab expert seamlessly at your side. DDL has over 35 years of first-hand experience navigating complex testing standards and medical device industry regulations – and holds a reputation for extraordinary, responsive service.
We specialize in testing the performance and safety of medical devices to prepare for regulatory submission, including Luer fittings, needles, syringes, catheters, and guidewires.
Medical device package testing
In addition, our package testing services ensure that packages are able to withstand the typical events associated with distribution, handling and storage without defect and loss of sterility. Major manufacturing companies around the world trust our processes and abilities to provide this critical testing for package validation.
Contact us for more information or to talk to an engineer.
Lab Testing Quality & Accreditations
Learn more about our commitment to quality.
DDL is an
ISO 17025
Accredited
& GMP Laboratory
ISO 80369 FAQs
ISO 80369 Part 7 is the replacement to ISO 594, the standard governing dimensions and performance requirements of Luer connectors. In this white paper, DDL provides responses to the most common questions customers have on ISO 80369.
Why DDL?
What we do at DDL matters to the lives of people. This is what drives us to become the most responsive and intelligently resourceful testing experts.
“I can’t thank you and your team enough for the outstanding service, support and well documented report that will be incorporated into our design file. I would strongly recommend DDL to any of our customer base for testing and assistance thru the luer transition stages.”